Browse By Unit
4.1 Open and Closed Systems: Energy
4 min read•june 18, 2024
Peter Apps
Daniella Garcia-Loos
Peter Apps
Daniella Garcia-Loos
Conservation Laws 👨💻
Certain quantities are conserved, in the sense that the changes of those quantities in a given system are always equal to the transfer of that quantity to or from the system by all possible interactions with other systems.
Energy is always conserved because it is a fundamental property of the universe that cannot be created or destroyed, only transformed from one form to another. This is known as the law of conservation of energy.
The law of conservation of energy states that the total amount of energy in a closed system remains constant, regardless of the changes that may occur within the system. This means that the energy that is lost by one part of the system must be gained by another part of the system.
The law of conservation of energy applies to all forms of energy, including kinetic energy (the energy of motion), potential energy (the energy of position or configuration), and thermal energy (the energy of heat).
Total Energy Changes 👨💻
Interactions with other objects or systems can change the total energy of a system.
Systems vs Objects 💡
A system is an object or a collection of objects. The objects are treated as having no internal structure.
In thermodynamics, a system is a region of the universe that is being studied or analyzed. A system can be either open or closed, depending on how it interacts with its surroundings.
An open system is a system that can exchange matter and energy with its surroundings. An open system can take in matter and energy from its surroundings, or it can release matter and energy into its surroundings. An open system is also called an exchange system.
A closed system, on the other hand, can only exchange energy with its surroundings, but not matter. An isolated system is a system that is isolated from its surroundings and cannot exchange matter or energy with them.
Force Interactions💡
An interaction can be either a force exerted by objects outside the system or the transfer of some quantity with objects outside the system.
What’s a System?
When we’re discussing energy and work, one of the main tasks to think about before doing any mathematics is to define the system of objects we are thinking about. A system simply put is the group of objects we plan to look at in a problem. Common systems include a ball and the earth, or a car and the road.
Using systems lets us define the difference between an internal force (one that is caused by a member of the system) and an external force (one that is caused by something outside of the system).
Work, Work, Work
Work in physics is a transfer of energy. Work occurs anytime there is an external net force applied to an object that moves it in a parallel direction to the force.
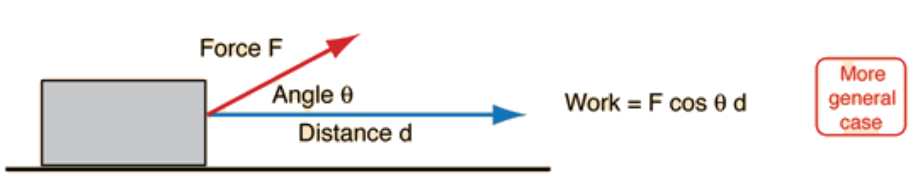
Image courtesy of __Hyperphysic__s.
In a general sense, work can be described by the equation W=Fdcos𝜃 where F is the applied force, d is the distance the object has the force applied over, and 𝜃 is the angle between the force and distance vectors.
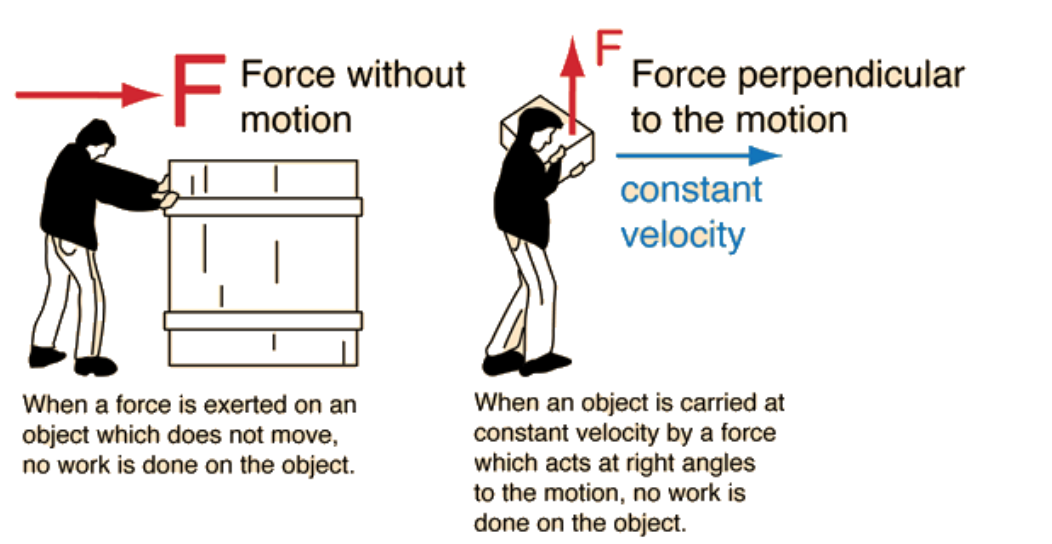
Image courtesy of Hyperphysics.
Similar to how displacement can be calculated from the area of a velocity vs time graph, work can be determined by using the area of a Force vs displacement graph. In AP 1, the area will always be able to be approximated by common geometric shapes (rectangles, triangles, etc). If you’re planning on taking AP C: Mech, you’ll also be able to use calculus to determine the work from non-geometric shapes.
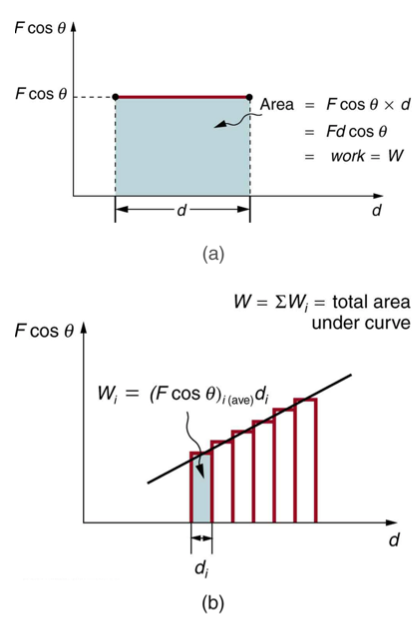
Image courtesy of opentextbook.
By convention, if work is done on the system it is considered to be positive work and will result in the total energy of the system increasing. Work done by the system is negative work and will remove energy from the system.
Power
Power is the rate at which Work is done on or by an object. It is described by the equation P = E/t, where E is the change in energy (Work), and t is the time needed for the change to occur.
Here are some key points about power:
- Power is a measure of the rate at which work is done or energy is transferred.
- Power is usually measured in watts (W), or joules per second (J/s).
- Power is calculated using the formula: Power = Work / Time
- Power is a scalar quantity, meaning it has only magnitude and no direction.
- Power can be positive or negative, depending on the direction of the work or energy transfer.
- Power is a measure of how quickly a task is completed or how fast energy is transferred. 🎥Watch: AP Physics 1 - Unit 4 Streams
<< Hide Menu
4.1 Open and Closed Systems: Energy
4 min read•june 18, 2024
Peter Apps
Daniella Garcia-Loos
Peter Apps
Daniella Garcia-Loos
Conservation Laws 👨💻
Certain quantities are conserved, in the sense that the changes of those quantities in a given system are always equal to the transfer of that quantity to or from the system by all possible interactions with other systems.
Energy is always conserved because it is a fundamental property of the universe that cannot be created or destroyed, only transformed from one form to another. This is known as the law of conservation of energy.
The law of conservation of energy states that the total amount of energy in a closed system remains constant, regardless of the changes that may occur within the system. This means that the energy that is lost by one part of the system must be gained by another part of the system.
The law of conservation of energy applies to all forms of energy, including kinetic energy (the energy of motion), potential energy (the energy of position or configuration), and thermal energy (the energy of heat).
Total Energy Changes 👨💻
Interactions with other objects or systems can change the total energy of a system.
Systems vs Objects 💡
A system is an object or a collection of objects. The objects are treated as having no internal structure.
In thermodynamics, a system is a region of the universe that is being studied or analyzed. A system can be either open or closed, depending on how it interacts with its surroundings.
An open system is a system that can exchange matter and energy with its surroundings. An open system can take in matter and energy from its surroundings, or it can release matter and energy into its surroundings. An open system is also called an exchange system.
A closed system, on the other hand, can only exchange energy with its surroundings, but not matter. An isolated system is a system that is isolated from its surroundings and cannot exchange matter or energy with them.
Force Interactions💡
An interaction can be either a force exerted by objects outside the system or the transfer of some quantity with objects outside the system.
What’s a System?
When we’re discussing energy and work, one of the main tasks to think about before doing any mathematics is to define the system of objects we are thinking about. A system simply put is the group of objects we plan to look at in a problem. Common systems include a ball and the earth, or a car and the road.
Using systems lets us define the difference between an internal force (one that is caused by a member of the system) and an external force (one that is caused by something outside of the system).
Work, Work, Work
Work in physics is a transfer of energy. Work occurs anytime there is an external net force applied to an object that moves it in a parallel direction to the force.

Image courtesy of __Hyperphysic__s.
In a general sense, work can be described by the equation W=Fdcos𝜃 where F is the applied force, d is the distance the object has the force applied over, and 𝜃 is the angle between the force and distance vectors.

Image courtesy of Hyperphysics.
Similar to how displacement can be calculated from the area of a velocity vs time graph, work can be determined by using the area of a Force vs displacement graph. In AP 1, the area will always be able to be approximated by common geometric shapes (rectangles, triangles, etc). If you’re planning on taking AP C: Mech, you’ll also be able to use calculus to determine the work from non-geometric shapes.

Image courtesy of opentextbook.
By convention, if work is done on the system it is considered to be positive work and will result in the total energy of the system increasing. Work done by the system is negative work and will remove energy from the system.
Power
Power is the rate at which Work is done on or by an object. It is described by the equation P = E/t, where E is the change in energy (Work), and t is the time needed for the change to occur.
Here are some key points about power:
- Power is a measure of the rate at which work is done or energy is transferred.
- Power is usually measured in watts (W), or joules per second (J/s).
- Power is calculated using the formula: Power = Work / Time
- Power is a scalar quantity, meaning it has only magnitude and no direction.
- Power can be positive or negative, depending on the direction of the work or energy transfer.
- Power is a measure of how quickly a task is completed or how fast energy is transferred. 🎥Watch: AP Physics 1 - Unit 4 Streams

© 2024 Fiveable Inc. All rights reserved.